Abstract
Introduction: Autologous stem cell transplantation (ASCT) is the standard of care and a mainstay in the management of eligible patients with multiple myeloma (MM) and remains relevant in the era of novel therapeutics. ASCT requires stem cell mobilization to collect ample stem cells to provide hematological recovery after myeloablative conditioning. Second ASCT (ASCT2) as salvage therapy in relapsed/refractory MM (RRMM) is a treatment option for patients who achieved disease control for 2 to 3 years after first ASCT (ASCT1). The reported difficulties in mobilizing and collecting additional stem cells following myeloablative ASCT1 have led to the currently adopted practice to collect and store adequate peripheral blood stem cells (PBSCs) for two transplants before ASCT. The preferred PBSC cell dose is 2 to 5 × 106 CD34+ cells per kilogram of recipient weight (CD34+ cells/kg) per transplant; however, with the influx of effective novel agents available for RRMM, the use of ASCT2 is declining. A retrospective cost utilization study showed that at a median follow-up of 3.5 years, only 3% of the cohort received ASCT2. The additional cost of collecting and storing these products is an average of 16,590 USD/patient. Given the limited use of salvage ASCT2, current practice of collecting PBSCs for two transplants should be re-evaluated and carefully selected case-by-case. We investigated potential strategies to reduce the financial burden of PBSC collection, processing, and storage by identifying a subgroup of patients who are less likely to receive an ASCT2, herein avoiding storing extra cells.
Materials and Methods: With Institutional Review Board approval, we performed a retrospective descriptive analysis of all MM patients who underwent frontline ASCT from 1/1/2012 to 6/23/2022. We excluded patients who had planned tandem transplants and those who underwent a single ASCT and had all cells infused with no remaining stored cells. We classified all patients who collected a sufficient cell dose for ASCT1 and ASCT2 into two cohorts according to the number of ASCT received; those who received ASCT1 and those who received ASCT1 and ASCT2.
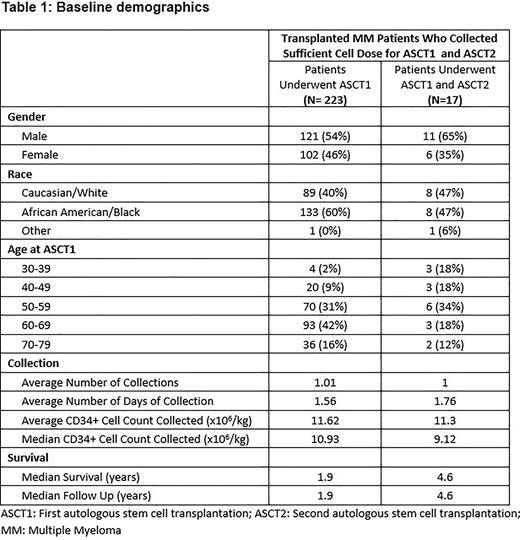
Results: We report the descriptive statistics of eligible MM patients from a single stem cell transplant center. Over a median follow-up of 2.0 years, we report findings of 240 patients who collected sufficient PBSCs for ASCT1 and ASCT2. Patients’ median age at PBSC collection was 61 (range 32-77). We classified our cohort by gender, age, race, and average number of days for PBSC collection. We aim to collect a cell dose of 10 x 106 CD34+ cells/kg. Our cohort included more males than females (132/108) and a higher prevalence of African Americans (59%) than Caucasians. Our study showed that only 7% of patients underwent ASCT2 (17/240) with average time between ASCT1 and ASCT2 of 2.9 years. We noticed that 88% (15/17) of patients who received ASCT1 and ASCT2 were ≤ 61 years old at the time of ASCT1 and two patients (12%) were ≥ 70 years. Additionally, a higher proportion of patients utilizing ASCT2 in the youngest age groups is noticeable. Six of 30 patients under 50 years and three of seven patients under 40 years of age (20% and 43%, respectively) underwent ASCT2. The data highlights that utilization of ASCT2 is less common in the elderly population; hence considering PBSC collection for one transplant among the elderly would be a cost-effective strategy. Most stem cell transplant centers inherited a strategy to collect PBSC 10 x 106 CD34+ cells/kg. We noticed that 102 patients required >1 day of PBSC collection to meet the collection goal. Of these patients, 35% were age 65 or older. If we were to change practice and consider PBSC collection for one transplant among the elderly, we could have reduced the number of collection days by 25 and reduced the associated costs of collection, processing, storage, and staffing, as well as space requirements for storage.
Conclusion: The utilization of ASCT2 in MM patients is decreasing with the influx of novel therapeutics. Potential strategies are needed to change the practice and reduce the additional financial burden of excess stem cell collection and storage. We suggest that elderly MM patients be considered for PBSC collections for a single transplant.
Disclosures
Kota:Xcenda: Honoraria; Incyte: Honoraria; Pfizer Inc: Honoraria, Research Funding; Ariad: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal